Demand for bio-based alternatives to traditional chemical ingredients is growing across every industry. Replacing chemical surfactants has become a key area of focus. These surfactants, also known surface-active agents, are a commonly used ingredient in products ranging from agriculture to construction to cleaning and even skincare.
Fermentation-produced biosurfactants, though not widely known, have emerged as a leading choice to lower the use of surfactants. Biosurfactants are a versatile category of surfactants. They’re garnering attention for their unique properties and eco-friendly nature.
Delve into the emerging world of biosurfactants and why they’re becoming a top choice in a wide range of applications, including agriculture, oil and gas, mining, consumer products, industrial and more.
What are Biosurfactants?
Biosurfactants represent a category of surfactants derived entirely from bio-based, renewable sources and manufactured through sustainable, non-chemical processes. To get more technical, biosurfactants are surface-active, amphiphilic compounds – a diverse group of molecules which consist of a polar (hydrophilic) head and a nonpolar (hydrophobic) tail.
How are biosurfactants made?
Biosurfactants can be derived from a diverse range of eco-friendly, organic materials. Unlike chemically produced or partially bio-based surfactants, biosurfactants are exclusively sourced from the biological activities of microorganisms. This ensures that their composition consists of 100% renewable carbon. Because biosurfactants are naturally produced by microorganisms, they a sustainable alternative to chemical surfactants (often derived from fossil fuels / petroleum) in various industries.
Biosurfactants are developed using sustainable production methods, with fermentation being a top choice. These remarkable compounds are synthesized through the metabolic processes of microorganisms, offering a greener alternative to traditional surfactants. While conventional surfactants often rely on hydrocarbon-based chemical reactions, biosurfactants are synthesized using fully renewable materials.
Unique properties of biosurfactants
Biosurfactants exhibit varying molecular structures, each tailored to specific applications. These molecules have enhanced surface and interfacial properties, unique characteristics and unmatched multifunctionality that allow them to maximize performance, value and cost-in-use savings.
Biosurfactants have a wide range of biological, physical and chemical functions including antibacterial, antiviral, antifungal, anti-inflammatory, anti-tumor, immunomodulating, chelating and many others.
Biosurfactants reduce surface tension
The unique structure of biosurfactants makes these compounds surface (interfacial) tension reducers. That means these biocompatible molecules exhibit surface activity at the interface of liquids and solids. That is why they are called biosurfactants (biological surface active agents). Surface tension can be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water molecules.
surface tension
Definition: the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water molecules.
For example, washing dirty clothes with only water would not be very effective, while adding a little bit of soap will help make the clothes cleaner. That is because soap is also a surfactant (which initially was made from amphiphilic saponins derived from plants). Soap allows both better absorption of particles from the liquid and better removal of particles from the surface.
The surface tension of water is 72 mN/m; a good biosurfactant can reduce this value to ≤ 35 mN/m, more than twice.
Biosurfactants have a smaller micelle size
The surface and interfacial tension reduction, and other properties of biosurfactants, are based on these molecules’ ability to form micelles: aggregations of biosurfactant molecules.
Biosurfactants have signficantly smaller micelle sizes, allowing them to outperform chemical surfactants. Typical surfactants typically have micelles averaging around 100 nanometers. Biosurfactants developed by Locus Fermentation Solutions (Locus FS) are only 3.1 nanometers. To put this into perspective, human DNA is 2.5 nanometers.
Biosurfactants have a lower CMC
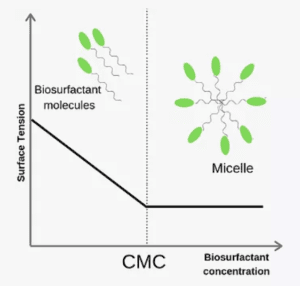
The concentration at which micelles start forming is called the critical micelle concentration (CMC). When surfactants reach CMC, they are able to greatly
reduce surface (interfacial) tension and therefore change the characteristics of the products containing these amphiphilic compounds (see figure on the right).
The benefit of such a mechanism is that once CMC is reached (for good biosurfactants this value is about 100 mg/L), the maximum effect is already reached and there is no necessity to use large amounts of a biosurfactant.
Biosurfactants can have a wide HLB range
Although named ”biosurfactants”, unlike many chemically synthesized surfactants, surface activity is not the only property of these molecules.
Biosurfactants, depending on the type, have solubilizing, emulsifying, dispersing, detergent and stabilizing properties, opening a wide spectrum for potential applications.
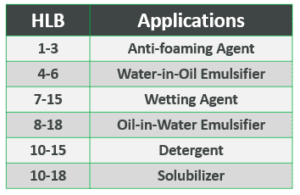
For example, emulsifying properties are determined by the HLB value. HLB is the ratio between the hydrophilic and hydrophobic parts of the molecule. The larger the hydrophilic portion is in comparison to the hydrophobic part, the higher the HLB is.
Depending on HLB value, the biosurfactant would be effective in different applications (see table).
Locus Fermentation Solutions offers biosurfactants with a range of HLB values, from low to high. The biomanufacturing company’s patented fermentation and downstream processing steps enables the ability to tune the hydrophilic-lipophilic balance (HLB) of the final biosurfactants.
Biosurfactants have more active sites per molecule
Biosurfactants typically have a more complex molecular structure than surfactants, which includes more active sites per molecule. This enables biosurfactants to have multiple functions within a product formulation.
Multifunctionality significantly impacts the performance and sustainability of an end product. By have multiple modes of action in a formulation, biosurfactants offer enhanced efficiency and lower usage rates.
Benefits of biosurfactants over surfactants
The unique properties of biosurfactants mentioned above, combined with their sustainable production, result in distinct advantages over chemical surfactants. This includes:
- lower toxicity
- lower carbon footprint
- higher activity levels
- better absorption
- high biodegradability
- better stability at extreme pH and salinity
See the table below for a full comparison of traditional surfactant properties compared to biosurfactant properties:
| Traditional Surfactants | Biosurfactants (Locus FS Sophorolipids) | |
| Surface Tension Reduction
Termed “surface tension”, is the attractive force between molecules present at the surface of a liquid. |
50-100+ ppm required
Water movement is often restricted |
> 2 ppm required
Enhances water mobility: |
| Multifunctionality
Ability to emulsify/demulsify, disperse, scour or provide other actions within a formulation |
1-2 typical functions | Perform multiple functions and can replace several legacy surfactants
Synergies boost performance of other water treatment chemicals |
| Micelle Size
Size of aggregate structures formed with surfactant molecules after a surface is saturated (measured in nanometers: nm) |
100 nm average size |
< 10 nm average size
Better Penetration: |
| Concentration
Inclusion rate needed to provide efficacious results |
High inclusion rates needed for optimal results | < 1/50 the inclusion rate of synthetic surfactants required |
Where are biosurfactants used?
The unique properties of biosurfactants make them valuable in applications across almost every industry. To fully leverage the capabilities of biosurfactants, it’s important to understand their structure, mechanisms of action and practical applications. That’s why companies around the world are turning to Locus Fermentation Solutions for expertise. Locus FS has successfully commercialized biosurfactant offerings in agriculture, oil & gas, mining, cleaning, home care, personal care, skincare and more.
READ MORE: Biosurfactant Applications: Powerful, Sustainable Solutions for Every Industry
Biosurfactants are organic compounds produced by microorganisms. These versatile molecules possess unique surface-active properties, making them invaluable across a spectrum of industries. Unlike their synthetic counterparts, biosurfactants are biodegradable, environmentally friendly and exhibit low toxicity. These bio-based agents are ushering in a new era of industry-wide eco-innovation.